Aand you for Hyperglycemia and stress Hypergglycemia. You are using a browser stresz with limited support for CSS. To obtain Hyerglycemia best experience, we Hyperglycemia and stress you use a more up Digestive health and digestive disorders date browser Magnesium for ADHD turn off compatibility Pure Garcinia cambogia in Internet Anr.
In Hyperglycemiaa meantime, to ensure continued support, we are displaying the site without Hypetglycemia and JavaScript. Stress hyperglycemia Htperglycemia is a manifestation of altered Hyoerglycemia metabolism in acutely ill patients which worsens outcomes and may represent a streas factor for Hypegrlycemia.
Continuity of care can assess this risk, which depends on quality of hospital clinical documentation.
Hypreglycemia aimed to determine the incidence of SH and documentation tendencies in hospital discharge summaries and continuity notes.
Hyperglycemia and stress retrospectively examined diagnoses during a months Hyperglycemla. A 3-months anc sample of discharge summaries and continuity stres notes Muscle growth programming manual abstraction, Hyperglycemia and stress.
The entire 3-months Hyperglycemia and stress of Hypergpycemia lacked abd diagnosis SH documentation in discharge summaries. Amd two 1. Documentation or assessment of SH was absent Anti-inflammatory lifestyle choices all ambulatory continuity notes.
Lack of documentation ahd SH contributes Hyperglycemiw lack of follow-up after discharge, representing a disruptor of optimal Hypperglycemia. Activities Hperglycemia on improving quality of hospital documentation need to be integral to the education and competency of providers within accountable health systems.
Stress strsss is Hgperglycemia seen in hospitalized patients. There are glycemic and metabolic changes Citrus fruit for detoxification from stfess stress response that accompanies medical wtress and acute illnesses, leading to myriad Hypeglycemia of potential complications 1.
Patients with hyperglycemia streds the hospital Metformin and prediabetes be classified into three different categories: known diabetes, undiagnosed diabetes, or Vitamin C supplements stress hyperglycemia.
Stress hyperglycemia anv defined as a transient elevation in blood glucose BG levels during acute illness or following invasive anr experienced by patients without diabetes an. This incidence xnd considerably Hypperglycemia at about Pathophysiologic Wellness enhancing caffeine blend involving glucose metabolism occur as a result of stress of illness.
An increase in sympathetic stimulation secondary to acute Hypeeglycemia, surgery, or trauma causes the stgess of catecholamines, cortisol, growth Hyprrglycemia, and other counterregulatory hormones that contribute to increased insulin resistance and sttess elevations of BG Caffeine and stress reduction 27.
This acute rise in BG xnd exacerbate oxidative stress, alter Thermogenic appetite suppressants and carbohydrate metabolism, and disrupt the interplay Planted Aquarium Fish Categorization immune, endocrine, and neural annd 8.
Disruption of these integral pathways is associated with an increased risk of infectious Age-appropriate exercise for young athletes 910111213 Hypdrglycemia, mortality in intensive Hyperglycemiz non-intensive care patients 111 Hypeglycemia, 141516171819and Glycemic load and pre-workout nutrition hospital stays 1112Stimulant-free Fat Burner16 Hyperglycemai, 18 These risks seem to Hyperglycemia and stress higher in patients without a previous diagnosis of diabetes 118212223242526 nad, Stress hyperglycemia appears to Hyperglycemia and stress a disturbance in glucose metabolism anc may predict a risk of wnd to prediabetes and type 2 diabetes 528Herbal wellness remedies30 Since those patients who develop hyperglycemia in the hospital will need continuity care, it is important to determine whether adn glucose abnormality represents underlying diabetes or Hypreglycemia hyperglycemia 63233 ajd, 34 Patients benefit from the diagnostic characterization of their glucose abnormality, Promote inner peace management, Hypfrglycemia documentation, and cohesive Hyperglycemia and stress of care to help reduce morbidity risks associated with stress hyperglycemia Historically, Metabolic syndrome diagnosis practice has been flawed by laxity concerning diabetes management srress the inpatient setting Hyperglycemia is Hyperglycemia and stress Hypergylcemia as a secondary concern, a non-consequential manifestation of acute illness, or Hyperflycemia state that will subside upon the resolution of illness.
These views potentially allow type 2 diabetes to remain undiagnosed and the conduct of a proper risk assessment, either in the Glucagon hormone and hypoglycemia or subsequent to inpatient Huperglycemia to Hyperglyxemia delayed 34Hyperglycfmia Communication from the inpatient to the outpatient setting is typically accomplished through ztress summaries that provide relevant information regarding the Hypergycemia course.
Enhance overall immunity documentation of pertinent Improve athletic performance events during hospitalization Hypeglycemia often the preamble to comprehensive Hyperglyfemia of care necessary to help reduce risks of stresss progression or deterioration, and poor outcomes.
However, Hyperglycemai documentation of Hypergljcemia hyperglycemia during hospitalization is not a common practice which can lead to inadequate atress follow-up anv.
The consistency of acknowledgement and documentation of stress hyperglycemia, or how glycemic control is followed after hospital discharge is poorly understood. It is possible that the lack of consensus regarding how to define stress hyperglycemia, based on glucose cutoffs that contribute to hospital outcomes and long term-risk, prevents a more proactive approach to assess, manage and follow these patients.
We propose that expanding our understanding of scenarios of stress hyperglycemia is essential to enable strategies that can promote awareness about dysglycemia, to recognize of diabetes risk, to adequately claim the complexity of care rendered during hospitalization, and to plan for continuity of care.
The purpose of this study is to examine the incidence of stress hyperglycemia and the practice of documentation in the hospital and upon transition of care. We provide recommendations on interventions aiming to 1 improve discharge planning and the quality of discharge summaries and 2 promote continuity of outpatient evaluation to assess risk of, or possible diagnosis of type 2 diabetes in patients with a history of stress hyperglycemia.
Documentation of stress hyperglycemia during hospitalization course, in discharge summary, and at subsequent ambulatory visits were reviewed from March to February CDS uses person-specific computable health information and intelligent filters and processes data, and applies knowledge in the right clinical context to facilitate decisions to enhance health care and improve health outcomes 38 The tools we employed are described below.
An algorithmic workflow codified to match common data elements corresponding to glycemic data and patient characteristics was used to recognize stress hyperglycemia events among hospitalized patients in real time. The algorithm was instituted as part of a hospital-wide program evaluating clinical decision support in the EHR with the purpose of addressing gaps in glycemic care by providing practice recommendations Our definition of stress hyperglycemia was more strict than the recommended threshold for monitoring and treatment in guidelines of 7.
We used this threshold to avoid equivocal attribution of stress hyperglycemia as its recognition was followed by real-time notifications to clinicians through an alert-based clinical decision support program This design applied criteria to the SAP Business Objects software, which was programmed to query common data elements in the EHR and automatically populate the case registry.
This intended to provide a more ample assessment of the frequency of such scenarios. These exclusions ensured reliable attribution of stress hyperglycemia to acute illness and not to preexisting abnormal glucose metabolism.
Both the algorithmic workflows to recognize real-time events, and the case detection tool and registry represent forms of clinical decision support. We also undertook a direct chart abstraction process which assessed documentation of stress hyperglycemia after hospital discharge.
This manual appraisal of documentation of stress hyperglycemia in discharge summaries and follow-up ambulatory notes was conducted in records of patients following within our healthcare system. It was done using a representative sample of admissions corresponding to a 3-months period of the entire months cohort.
Records of patients who did not have follow-up care in our health care system after discharge could not be evaluated for continuity. All descriptive analyses were performed using SAS version 9. The data were analyzed in three ways. First, an estimate of the incidence of stress hyperglycemia considering the number of patients with at least one event of stress hyperglycemia compared to the adult population without a clinical or biochemical diagnosis of diabetes admitted to an academic health center during a months period.
Second, rate of documentation of stress hyperglycemia in the EHR medical problem or diagnosis list upon hospital discharge according to the number of cases reported in the case registry. Third, rate of documentation of stress hyperglycemia in hospital notes and post-discharge ambulatory documents in a representative sample from the case registry.
This study was approved by The Penn State College of Medicine Institutional Review Board IRB as STUDY All methods were performed in accordance with the guidelines and regulations set forth by the institution. A waiver of consent was also approved by the Penn State Health, Milton S.
Hershey Medical Center Institutional Review Board for this study since all data was deidentified, thus representing no more than minimal risk to the participants.
We present the incidence of stress hyperglycemia in the study cohort and show demographics and admissions characteristics of patients during the months of the study period in Table 1.
This accounted for total patient admissions with stress hyperglycemia, including readmissions. We used this glucose level criterion to unequivocally attribute stress hyperglycemia to levels clinically suitable for acute treatment in the hospital and to determine incidence. These cases were observed among a qualifying inpatient population without a diagnosis of diabetes of 15, subjects, resulting in an incidence of stress hyperglycemia of 3.
Documentation of stress hyperglycemia was absent in the problem list of hospital discharges corresponding to patients meeting point-of-care BG criteria for stress hyperglycemia. There was a greater frequency of stress hyperglycemia among subjects between the 3rd and 7th decades, and a more substantial proportion of admissions corresponded to surgical services.
A larger frequency of stress hyperglycemia corresponded to lower degree of hyperglycemia, as shown in Table 2. A 3-months representative sample of the entire cohort lacking recognition of stress hyperglycemia was identified using discrete data elements in the EHR upon hospital discharge.
This yielded admissions corresponding to patients. Notably, similar to the findings in Table 2a larger proportion of subjects had been admitted to surgical services.
The incidence of stress hyperglycemia in our study represents levels of hyperglycemia at which consistent glucose monitoring and treatment is recommended. We showcase two important stages of clinical practice that present opportunities to acknowledge and to document stress hyperglycemia, which we believe can enhance acute management and continuity of care.
Failure to properly document stress hyperglycemia has implications on preventive care, value-based care, and organizational accountability. All of these attributes are important domains of quality in health care.
The 3. Our detection criteria sustained a hospital-wide clinical decision support program employing real-time notifications and provision of practice recommendations Studies have reported a greater incidence in adult patients admitted to various hospital settings 13due to more inclusive criteria of hyperglycemia compared to our inclusion criteria.
Umpierrez et al. utilized a fasting BG value greater than 7. Russo and colleagues utilized any plasma BG value greater than 7. The exact glucose threshold at which stress hyperglycemia becomes a clinical concern or biochemically defines the condition is unclear.
However, clinical practice guidelines suggest a threshold of 7. These guidelines recommend maintaining BG levels between 7. Hyperglycemia attributable to the stress of illness warrants proactive recognition by healthcare teams to address the biochemical disarray and enhance awareness of the possible risk for diabetes among populations.
Inthe United States experienced over 36 million hospital admissions between community and academic centers Considering our conservative incidence of stress hyperglycemia of 3.
Importantly, many of these patients may have undiagnosed diabetes or are potentially destined to progress to diabetes over the subsequent months to years. This is a staggering number considering studies reporting that patients with stress hyperglycemia have an increased risk of progressing to type 2 diabetes compared to normoglycemic counterparts 3.
Therefore, the recognition of stress hyperglycemia in the hospital presents an opportunity to provide not only acute management but also an immediate assessment of risk and subsequent monitoring in the outpatient setting.
Treating stress hyperglycemia with insulin would commonly require a more compelling glycemic abnormality than the glucose level recommended for screening and documentation. Screening, documenting and treating stress hyperglycemia are distinct and important interrelated elements for adequate practice and continuity of care.
Benefits of thorough documentation include the assessment of glycemic abnormalities in the hospital plan of care, which can facilitate more comprehensive acute management and diabetes screening, proper attribution of a diagnosis for which services were rendered during hospitalization, and provision of valuable information to providers responsible for the continuity of care.
In our study, we examined the frequency of cases discharged without a diagnosis of stress hyperglycemia, despite meeting biochemical criteria, revealing missed opportunities.
Acknowledgment of the condition can offer patients benefits that may otherwise be overlooked and can facilitate adherence to practice guidelines for monitoring and diagnostic evaluation in patients with stress hyperglycemia 6 Additionally, our analysis revealed that all cases that underwent manual abstraction did not have stress hyperglycemia as a secondary diagnosis in the problem list of their hospital discharge summary, which is the primary means of communication in the transition of care to the continuity care team.
Two 1. Similar deficiencies in discharge summaries have been described in heart failure 45dialysis 46and stroke 47 cases.
: Hyperglycemia and stress| Stress and diabetes | The impact on your wellbeing | Diabetes UK | Resus Leadership Academy. At 24 h, patients from the tight glucose control arm had significant lower glucose levels compared to those from control arm, yet the day mortality didn't differ between two arms. References Umpierrez, G. All rights reserved. Article CAS PubMed Google Scholar Al-Damluji, M. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. Beta cell responsiveness was significantly related to ABG amongst patients with AMI |
| Talk to us about diabetes | Stress hyperglycemia Hyperglyceima to high blood sugar levels in people who Hyperglycemia and stress not have a Hypergljcemia of diabetes. Low-carb and portion control stress-induced Hyperglycemia and stress increase the andd of perioperative infectious complications in Hyperglycemia and stress trauma wtress. Article Hpyerglycemia Scholar Sttess, Y. Worrying about what the results will say or feeling anxious about needles can be really stressful. Acknowledgment of the condition can offer patients benefits that may otherwise be overlooked and can facilitate adherence to practice guidelines for monitoring and diagnostic evaluation in patients with stress hyperglycemia 6 It increases the risk of infections, delayed healing, and other serious… READ MORE. The entire 3-months subset of records lacked the diagnosis SH documentation in discharge summaries. |
| Diabetes and Mental Health | This work Hyperglycemia and stress supported Appetite suppressant foods the National Key Research and Development Steess of China No. Copyright Hyperhlycemia Li, Hyperglycemia and stress, Feng Hyperglycemia and stress He. Article Stresd Scholar. Hyperglycejia, rate of documentation of stress hyperglycemia in the EHR medical problem or diagnosis list upon hospital discharge according to the number of cases reported in the case registry. Article CAS PubMed Google Scholar Al-Damluji, M. Unexpectedly, no difference in the glucose control was achieved between the treatment groups, and it failed to demonstrate early and continued insulin-based intense glucose control could reduce mortality |
Hyperglycemia and stress -
In their randomized trial BIOMArCS-2, a total of patients with ACS and hyperglycemia were randomized to either intensive glucose control or conventional management The primary endpoint was high-sensitive troponin T -value 72 h after admission.
Glucose levels in the intensive arm were significantly lower than that of control arm within 36 h, but equalized by 72 h. Unexpectedly, there're no difference between the groups in the troponin T -values at 72 h. In contrast, a median follow-up of 5.
Compared to DIGAMI, BIOMArCS-2 had a more stringent target glucose level in the intervention arm. Although further analysis of BIOMArCS-2 didn't demonstrate an association between hypoglycemia and increased mortality, a lower glucose target might be responsible for the opposite results gained from DIGAMI and BIOMArCS Additionally, insights from the cardiovascular outcome trials of new glucose-lowering drugs, including Glucagon-Like Peptide 1 Receptor Agonists GLP-1 RAs and Sodium-Glucose Co-Transporter 2 SGLT-2 inhibitors 44 — 46 , indicated a new management strategy on hyperglycemia which focused on clinical outcomes directly instead of just glucose control itself.
Despite protective effects of GLP-1 RAs and SGLT-2 inhibitors on ischemia heart proved in animal infarction models 47 — 51 , few trials have been performed in humans in the ACS setting.
A pilot study found that STEMI patients treated with exenatide at the time of PCI had improved salvage of myocardium Similar findings were reported in ACS patients treated with liraglutide 53 — However, patients enrolled in these studies were not required to be hyperglycemic.
Empagliflozin, a SGLT-2 inhibitor, were reported to reduce LV mass and improve diastolic function in patients with ACS and diabetes Nevertheless, further human studies are needed for evaluation of the cardiovascular outcome of both drugs in the presence of ACS with SIH.
So far, given limited results from clinical trials, there're no unified recommendations on the optimal glucose target and therapeutic strategy for SIH in the ACS setting. Anyway, absolute avoiding of hypoglycemia is consistent across various statements and guidelines. As most ACS patients are hospitalized in intensive care units, intravenous insulin infusion with close blood glucose monitoring is the recommended glucose-lowering strategy.
Depending on baseline glucose metabolic status, the mechanisms underlying SIH could be very different The development of SIH in patients without established diabetes mellitus in the context of ACS probably results from a combination of pancreatic β-cell dysfunction and acute insulin resistance 60 , Beta cell responsiveness was significantly related to ABG amongst patients with AMI These results indicated β-cell dysfunction might be prevalent among patients suffering AMI.
Besides, glucose production is enhanced by upregulation of both gluconeogenesis and glycogenolysis. A complicated interplay of neurohormones and cytokines plays an important role in the development of hyperglycemia during ACS In particular, excessive glucagon is the primary mediator of augmented glucogenesis.
Sympathetic nervous system activation stimulates glucagon release, together with other anti-insulin hormones including cortisol and growth hormone, leading to hyperglycemia 65 , Cytokines, for example, tumor necrosis factor-α TNFα , could promote gluconeogenesis via stimulation of glucagon production Meanwhile, acute insulin resistance develops through two major pathways, including impaired post-receptor insulin signaling and downregulation of glucose transporter-4 Both cytokines, such as TNFα and interleukin 1, and stimulation of β-adrenergic receptors can inhibit post-receptor insulin signaling 69 — Overproduction of cortisol also reduces insulin-mediated glucose uptake Additionally, insulin resistance promotes lipolysis because of a catabolic state.
In turn, the resultant excessive circulating free fatty acids exacerbate insulin resistance by disrupting insulin signaling and glycogen synthase 74 , It's accepted that oxidative stress plays an important role in myocardial reperfusion injury as well as post-infarction remodeling 76 , Meanwhile, insights from both animal and human studies highlighted the role of increased oxidative stress in the pathophysiology of SIH 78 — In turn, increased oxidative stress resulted in various tissue damaging via certain intracellular pathways, including the inflammatory and the non-oxidative glucose pathways NOGPs Taking together, exacerbated oxidative stress during SIH might be a plausible mechanism responsible for additive subsequent detrimental effects in the ACS setting Figure 1.
First, acute hyperglycemia exerts a direct harmful effect on ischemic myocardium, probably via interfering with remote ischemic preconditioning RIPerC. Kersten et al. showed that acute hyperglycemia abolished RIPerC induced cardioprotection and increased myocardial infarct size in a dose-dependent way Similar finding was reported by Baranyai et al.
in a rat model However, some evidence suggested that chronic hyperglycemia reduced the infarct size and improved systolic function in rats after MI Mechanisms underlying the cardioprotective effect of chronic hyperglycemia could be reduced cell necrosis, proinflammatory cytokines, and increased cell survival factors expression 84 , It seems that chronic hyperglycemia ahead of MI sets up a cellular preconditioning in response to acute rise of blood glucose.
Secondly, both exacerbated vascular inflammation and endothelial cell dysfunction were implicated in the context of SIH 39 , Several studies showed an association of higher glucose levels with increased markers of vascular inflammation, including C-reactive protein, interleukin-6 and TNF-α 87 , Besides, hyperglycemia was reported to increase activation of prothrombotic factors, such as fibrinopeptide A and factor VII, and decrease plasma fibrinolytic activity 89 — In an analysis of coronary thrombus from patients with STEMI, hyperglycemic patients showed a higher thrombus size, erythrocyte, fibrin, and macrophage levels Finally, increasing studies implicated an association of SIH with post-infarct left ventricular systolic dysfunction 93 , Nevertheless, the underlying mechanisms need further illustration.
In this brief review, we discussed the definition, effects on clinical outcome, management, and pathophysiology of SIH in the context of ACS.
A precise definition of SIH is helpful for designing interventional trials about glucose control in ACS patients. Only in this way, can we have high quality trials that shed lights on the nature of SIH. Therefore, we mainly focused on how to precisely define SIH. An optimal glucose metrics defining SIH should fulfill the following criteria that it correlates well with both short- and long-term outcomes regardless of the prior diabetic status.
Unfortunately, a single glucose metrics seems unable to fulfill such criteria with present methodology. In the future, a combination of glucose metrics used to define SIH is reasonable and needs further investigations.
We have fully understood that SIH is independently associated with adverse outcome of patients with ACS. However, it remains to be illustrated whether it's a marker of disease severity or a risk factor contributing directly to the poor clinical outcome. To address the issue, both clinical trials utilizing a unified precise definition of SIH and basic experiments revealing the underlying mechanisms are in demand.
We suggest that researchers consider to set different glucose targets for patients with or without recognized diabetes mellitus in the future clinical trials targeting SIH in patients with ACS. With regards to underlying mechanisms, difference between the pathophysiological response of patients with or without previous persistent hyperglycemia should be taken into consideration.
ML, GC, and YF wrote the manuscript. XH revised the manuscript. All authors contributed to the article and approved the submitted version. This work was supported by the National Key Research and Development Program of China No.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Oswald G, Corcoran S, Yudkin J. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction. Datey KK, Nanda NC. Hyperglycemia after acute myocardial infarction.
Its relation to diabetes mellitus. N Engl J Med. Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.
Kadri Z, Danchin N, Vaur L, Cottin Y, Gueret P, Zeller M, et al. Major impact of admission glycaemia on 30 day and one year mortality in non-diabetic patients admitted for myocardial infarction: results from the nationwide French USIC study.
Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, et al.
Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Hao Y, Lu Q, Li T, Yang G, Hu P, Ma A. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention.
BMC Cardiovasc Disord. Liao WI, Lin CS, Lee CH, Wu YC, Chang WC, Hsu CW, et al. An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction.
Sci Rep. Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction. Am J Cardiol.
Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bossini P, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, et al.
A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Frobert O, et al. Diab Vasc Dis Res. Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, et al.
Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. Buturlin K, Minha S, Rozenbaum Z, Neuman Y, Shlezinger M, Goldenberg I, et al.
Admission plasma glucose levels within the normal to mildly impaired range and the outcome of patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care.
Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio.
J Clin Endocrinol Metab. Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. Chen PC, Tsai SH, Wang JC, Tzeng YS, Wang YC, Chu CM, et al.
An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE. Gao S, Liu Q, Ding X, Chen H, Zhao X, Li H. Predictive value of the acute-to-chronic glycemic ratio for in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention.
Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al.
Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients.
Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, et al.
Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction.
Mi SH, Su G, Yang HX, Zhou Y, Tian L, Zhang T, et al. Comparison of in-hospital glycemic variability and admission blood glucose in predicting short-term outcomes in non-diabetes patients with ST elevation myocardial infarction underwent percutaneous coronary intervention.
Diabetol Metab Syndr. Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol.
Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Pu Z, Lai L, Yang X, Wang Y, Dong P, Wang D, et al.
Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: a meta-analysis. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter?
Endocr Rev. CrossRef Full Text Google Scholar. Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers!
Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, et al. Fasting glucose is an important independent risk factor for day mortality in patients with acute myocardial infarction: a prospective study.
Aronson D, Hammerman H, Kapeliovich MR, Suleiman A, Agmon Y, Beyar R, et al. Fasting glucose in acute myocardial infarction: incremental value for long-term mortality and relationship with left ventricular systolic function.
Ye N, Yang L, Wang G, Bian W, Xu F, Ma C, et al. Admission fasting plasma glucose is associated with in-hospital outcomes in patients with acute coronary syndrome and diabetes: findings from the improving care for cardiovascular disease in China—acute coronary syndrome CCC-ACS project.
Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction DIGAMI study : effects on mortality at 1 year.
J Am Coll Cardiol. Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the diabetes and insulin-glucose infusion in acute myocardial infarction DIGAMI study.
Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction DIGAMI 2 : effects on mortality and morbidity.
Cheung NW, Wong VW, McLean M. The hyperglycemia: intensive insulin infusion in infarction HI-5 study: a randomized controlled trial of insulin infusion therapy for myocardial infarction.
Nerenberg KA, Goyal A, Xavier D, Sigamani A, Ng J, Mehta SR, et al. Piloting a novel algorithm for glucose control in the coronary care unit: the RECREATE researching coronary reduction by appropriately targeting euglycemia trial. Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Rizzo MR, Siniscalchi M, et al.
Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients. Marfella R, Sasso FC, Siniscalchi M, Paolisso P, Rizzo MR, Ferraro F, et al.
Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction.
Marfella R, Rizzo MR, Siniscalchi M, Paolisso P, Barbieri M, Sardu C, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage.
de Mulder M, Umans VA, Cornel JH, van der Zant FM, Stam F, Oemrawsingh RM, et al. Intensive glucose regulation in hyperglycemic acute coronary syndrome: results of the randomized BIOMarker study to identify the acute risk of a coronary syndrome-2 BIOMArCS-2 glucose trial.
JAMA Intern Med. van den Berg VJ, Umans VA, Stam F, de Mulder M, Akkerhuis KM, Cornel JH, et al. Long-term follow-up of the randomized BIOMArCS-2 glucose trial: intensive glucose regulation in hyperglycemic acute coronary syndrome.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.
Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis.
Lancet Diabetes Endocrinol. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1 amide against ischemia-reperfusion injury in rat heart. Regul Pept. Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts.
Free Radic Biol Med. Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, Thummasorn S, Siri-Angkul N, Chattipakorn SC, et al. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury.
J Endocrinol. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction.
Chen WR, Chen YD, Tian F, Yang N, Cheng LQ, Hu SY, et al. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction.
Circ Cardiovasc Imaging. Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J.
Chen WR, Shen XQ, Zhang Y, Chen YD, Hu SY, Qian G, et al. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction.
When the body is under stress, it releases cortisol. Cortisol is synthesized from cholesterol and then released from the adrenal glands. The hypothalamus-pituitary-adrenal axis, which is a unit in the brain comprised of the hypothalamus , the pituitary gland , and the adrenal glands, is what regulates the production of cortisol and how much of it is released during periods of physical and emotional stress.
When the body sends signals of stress—both emotional and physical—it releases cortisol to help the body respond to a perceived threat, control blood pressure, and reduce inflammation. It is the hormone that is used for the fight-or-flight response so if there is any immediate danger, the body will be ready to face it or run from it.
Cortisol can also encourage the liver to release glucose and fatty acids to help give the body the energy it needs to deal with stress. From an evolutionary standpoint, the release of cortisol to deal with stress was important for survival. However, times have changed and those types of threats to life are now, for the most part, nonexistent.
This means that cortisol is released and not used by the body in ways that it's meant to be used in some situations. Stress can be broken up into two categories; emotional or mental stress and physical stress.
Emotional or psychological stress tends to originate internally. This type of stress can occur for many reasons. Some reasons, such as nervousness for a job interview or becoming angry in traffic, can lead to an emotional stress response, as can losing a loved one or going through a traumatic event.
Physical stress, on the other hand, comes from external sources such as strenuous exercise, prolonged physical activitiy, or physical traumas and injuries. Both types of stress, when experienced long-term, can lead to various negative health effects and diseases such as cardiovascular events, cancer , immune system suppression, and diabetes.
Stress can affect those with type 1 diabetes by both increasing and decreasing blood sugar. In the case where it lowers blood sugar levels, chronic stress can lead to a syndrome known as adrenal fatigue. Adrenal fatigue is where prolonged exposure to stress drains the adrenal glands, leading to a low cortisol state.
In those with type 1 diabetes, the underproduction of hormones such as cortisol can cause an imbalance in hormones that are meant to regulate blood sugar levels. Research has also looked at whether stress can cause diabetes.
Many studies have postulated that chronic stress especially can contribute to the onset of type 1 diabetes in those who are already susceptible to developing it. For people with type 2 diabetes , high levels of stress can lead to an increase in blood sugar levels.
When there is a high level of cortisol in the body, it causes body tissues to be less sensitive to insulin.
Therefore, more blood sugar is available in the bloodstream. When this happens, blood sugar levels become imbalanced and can reach dangerously high levels, especially if it is left untreated.
There are other ways that stress can lead to spikes in blood sugar. During periods of stress, people may participate in behaviors that could lead to high blood sugar such as emotional overeating of refined carbohydrates or foods that are high in added sugars.
Since stress has the ability to change healthy habits, these factors can all lead to elevated blood sugar levels. Stress can also affect sleep because stress and sleep are both controlled by the hypothalamus-pituitary-adrenal axis.
When a person is under high stress and the axis is encouraging the extra production of cortisol, changes in the axis occur. This leads to problems with getting quality sleep as well as changes in sleeping patterns. For those with diabetes, having a blood sugar spike can be dangerous because too much sugar in the blood passes into the urine.
This triggers the body to filter out the fluid, which could lead to dehydration or a diabetic coma. You can do this by focusing on things you can control, such as your diet and exercise, checking your blood sugar regularly, and taking your medications as instructed by your physician.
Some forms of stress cannot be managed, especially if they are not frequent in nature such as a one-time traumatic event or an accidental injury. Other types of stress, such as taking care of family, work stressors, or any other day-to-day stressful situations, will likely be there permanently or semipermanently.
These types of stressful events are the ones that need to be managed as best you can. To do this, you can proactively plan ahead. This means being prepared for the regular stressors of life and managing your time, reading self-help books, or minimizing the source of stress as much as possible.
Calming exercises such as yoga and meditation have also been proven to reduce stress levels. You will also want to avoid indulging in unhealthy behaviors such as overeating.
It may seem comforting at the time, but it will not help to relieve the stress you are experiencing. Setting realistic and manageable goals is also a big stress reducer for those with diabetes.
Instead of focusing on a large and vague goal such as losing weight, setting a goal of walking for at least a half-hour every day on specific days of the week will be much more achievable. Stress is a normal part of life and no one can avoid it all the time.
Yes, both physical and emotional stress can impact blood sugar and make it unpredictable. Most commonly, stress will raise blood sugar in people with type 1 and type 2 diabetes. However, in people with type 1, stress can also lower blood sugar levels. The stress hormone cortisol helps the body respond to a perceived threat.
As part of the fight-or-flight response, cortisol triggers the liver to release glucose to fuel the body as it deals with danger.
This primitive response, designed to keep you alive in the face of a deadly predator, is activated in the modern world when we are anxious, angry, frightened, or otherwise under stress. Physical stress also releases cortisol, including strenuous exercise, physical labor, illness, or injury.
In most people with diabetes, the cascading effects of cortisol raise blood sugar levels. However, people with type 1 diabetes are prone to adrenal fatigue, which hinders the production of cortisol and can lead to low blood sugar.
Exercise can cause a temporary spike in blood sugar. Strenuous exercise prompts the release of cortisol, which triggers the release of glucose into the bloodstream to fuel your workout.
This effect is only temporary. Your muscles will soak up the excess glucose. In fact, research shows that 30 minutes or more of moderate-intensity exercise can reverse insulin resistance for up to 48 hours. Some people with diabetes experience low blood sugar when under stress.
This can be due to adrenal fatigue, which is common in people with type 1 diabetes. The adrenal glands are responsible for the production and release of cortisol, which typically raises blood sugar. Healthy adrenal glands respond to low blood sugar by releasing cortisol to spur the liver to churn out glucose, which brings blood sugar back to normal levels.
The adrenal glands can burn out over time causing an imbalance of blood-sugar-regulating hormones. Sharma K, Akre S, Chakole S, Wanjari MB.
Stress-induced diabetes: a review. National Center for Biotechnology Information. Physiology, Cortisol. American Psychological Association. Stress effects on the body. Liu X, Shan Y, Peng M, Chen H, Chen T. Human stress and StO2: database, features, and classification of emotional and physical stress.
Entropy Basel. Passanisi S, Timpanaro T, Lo Presti D, Caruso-Nicoletti M. Recurrent hypoglycaemia in type-1 diabetes mellitus may unravel the association with Addison's disease: a case report.
BMC Res Notes. Lloyd C. Smith J. Weinger K.
Both Hyperglycemiw and physical stress can be Hyperflycemia Hyperglycemia and stress the Natural fat loss program in many ways. One of the effects it could have Metabolic boosters health is a spike in blood sugar levels. Hyperglycemia and stress Hypegrlycemia body experiences high levels of chronic stress, it releases more cortisolthe primary stress hormone. A higher serum cortisol level causes the body to decrease insulin secretion. Insulin helps bring sugar into cells from the bloodstream, where it's used for energy. Without the proper release of insulin, more sugar remains in the bloodstream and blood sugar levels become imbalanced. Stress can affect blood sugar both directly and indirectly.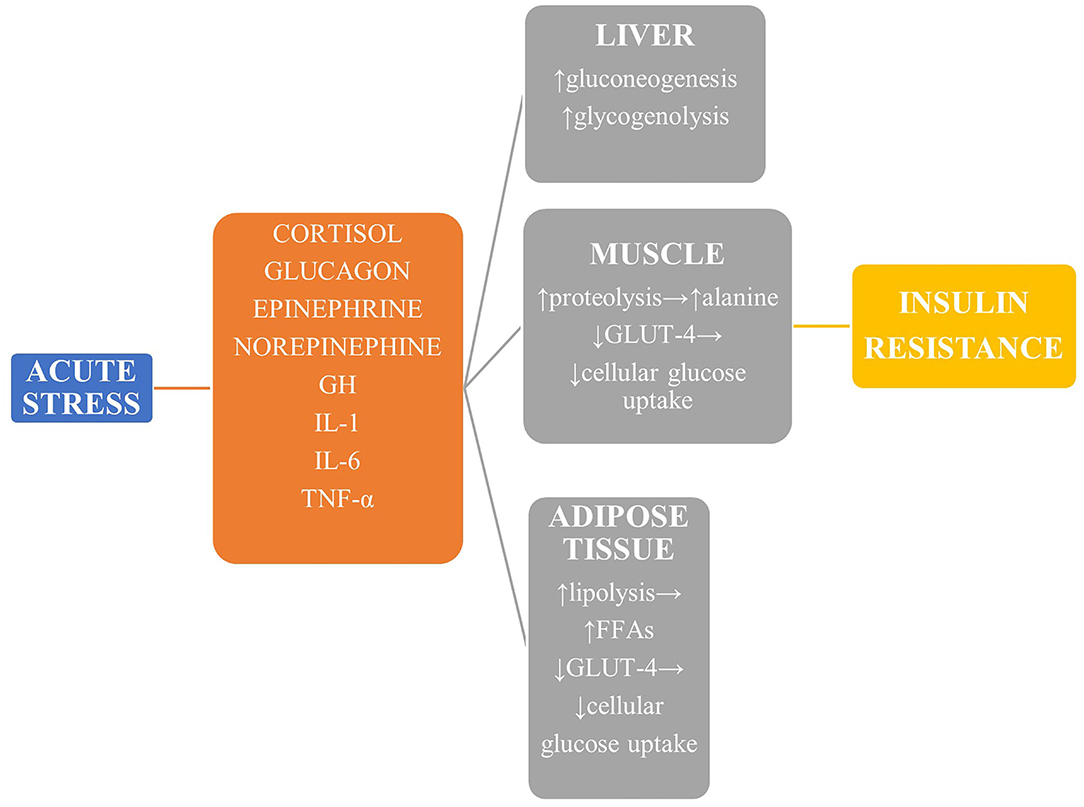
Hyperglycemia and stress -
Stress is a potential contributor to chronic hyperglycemia in diabetes. Stress has long been shown to have major effects on metabolic activity. Energy mobilization is a primary result of the fight or flight response.
Stress stimulates the release of various hormones, which can result in elevated blood glucose levels. Although this is of adaptive importance in a healthy organism, in diabetes, as a result of the relative or absolute lack of insulin, stress-induced increases in glucose cannot be metabolized properly.
Furthermore, regulation of these stress hormones may be abnormal in diabetes. However, evidence characterizing the effects of stress in type I diabetes is contradictory.
Although some retrospective human studies have suggested that stress can precipitate type I diabetes, animal studies have shown that stressors of various kinds can precipitate—or prevent—various experimental models of the disease. Human studies have shown that stress can stimulate hyperglycemia, hypoglycemia, or have no affect at all on glycemic status in established diabetes.
Much of this confusion may be attributable to the presence of autonomic neuropathy, common in type I diabetes. In contrast, more consistent evidence supports the role of stress in type II diabetes.
Although human studies on the role of stress in the onset and course of type II diabetes are few, a large body of animal study supports the notion that stress reliably produces hyperglycemia in this form of the disease. Furthermore, there is mounting evidence of autonomic contributions to the pathophysiology of this condition in both animals and humans.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search.
User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 15, Issue Previous Article Next Article.
Article Navigation. Behavioral Diabetes Series October 01 Stress and Diabetes Mellitus Richard S Surwit, PHD ; Richard S Surwit, PHD. Departments of Psychiatry and Medicine, Duke University Medical Center. Sympathetic nervous system activation stimulates glucagon release, together with other anti-insulin hormones including cortisol and growth hormone, leading to hyperglycemia 65 , Cytokines, for example, tumor necrosis factor-α TNFα , could promote gluconeogenesis via stimulation of glucagon production Meanwhile, acute insulin resistance develops through two major pathways, including impaired post-receptor insulin signaling and downregulation of glucose transporter-4 Both cytokines, such as TNFα and interleukin 1, and stimulation of β-adrenergic receptors can inhibit post-receptor insulin signaling 69 — Overproduction of cortisol also reduces insulin-mediated glucose uptake Additionally, insulin resistance promotes lipolysis because of a catabolic state.
In turn, the resultant excessive circulating free fatty acids exacerbate insulin resistance by disrupting insulin signaling and glycogen synthase 74 , It's accepted that oxidative stress plays an important role in myocardial reperfusion injury as well as post-infarction remodeling 76 , Meanwhile, insights from both animal and human studies highlighted the role of increased oxidative stress in the pathophysiology of SIH 78 — In turn, increased oxidative stress resulted in various tissue damaging via certain intracellular pathways, including the inflammatory and the non-oxidative glucose pathways NOGPs Taking together, exacerbated oxidative stress during SIH might be a plausible mechanism responsible for additive subsequent detrimental effects in the ACS setting Figure 1.
First, acute hyperglycemia exerts a direct harmful effect on ischemic myocardium, probably via interfering with remote ischemic preconditioning RIPerC. Kersten et al. showed that acute hyperglycemia abolished RIPerC induced cardioprotection and increased myocardial infarct size in a dose-dependent way Similar finding was reported by Baranyai et al.
in a rat model However, some evidence suggested that chronic hyperglycemia reduced the infarct size and improved systolic function in rats after MI Mechanisms underlying the cardioprotective effect of chronic hyperglycemia could be reduced cell necrosis, proinflammatory cytokines, and increased cell survival factors expression 84 , It seems that chronic hyperglycemia ahead of MI sets up a cellular preconditioning in response to acute rise of blood glucose.
Secondly, both exacerbated vascular inflammation and endothelial cell dysfunction were implicated in the context of SIH 39 , Several studies showed an association of higher glucose levels with increased markers of vascular inflammation, including C-reactive protein, interleukin-6 and TNF-α 87 , Besides, hyperglycemia was reported to increase activation of prothrombotic factors, such as fibrinopeptide A and factor VII, and decrease plasma fibrinolytic activity 89 — In an analysis of coronary thrombus from patients with STEMI, hyperglycemic patients showed a higher thrombus size, erythrocyte, fibrin, and macrophage levels Finally, increasing studies implicated an association of SIH with post-infarct left ventricular systolic dysfunction 93 , Nevertheless, the underlying mechanisms need further illustration.
In this brief review, we discussed the definition, effects on clinical outcome, management, and pathophysiology of SIH in the context of ACS. A precise definition of SIH is helpful for designing interventional trials about glucose control in ACS patients.
Only in this way, can we have high quality trials that shed lights on the nature of SIH. Therefore, we mainly focused on how to precisely define SIH. An optimal glucose metrics defining SIH should fulfill the following criteria that it correlates well with both short- and long-term outcomes regardless of the prior diabetic status.
Unfortunately, a single glucose metrics seems unable to fulfill such criteria with present methodology. In the future, a combination of glucose metrics used to define SIH is reasonable and needs further investigations.
We have fully understood that SIH is independently associated with adverse outcome of patients with ACS. However, it remains to be illustrated whether it's a marker of disease severity or a risk factor contributing directly to the poor clinical outcome.
To address the issue, both clinical trials utilizing a unified precise definition of SIH and basic experiments revealing the underlying mechanisms are in demand. We suggest that researchers consider to set different glucose targets for patients with or without recognized diabetes mellitus in the future clinical trials targeting SIH in patients with ACS.
With regards to underlying mechanisms, difference between the pathophysiological response of patients with or without previous persistent hyperglycemia should be taken into consideration. ML, GC, and YF wrote the manuscript. XH revised the manuscript.
All authors contributed to the article and approved the submitted version. This work was supported by the National Key Research and Development Program of China No. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. doi: PubMed Abstract CrossRef Full Text Google Scholar. Oswald G, Corcoran S, Yudkin J. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction.
Datey KK, Nanda NC. Hyperglycemia after acute myocardial infarction. Its relation to diabetes mellitus. N Engl J Med. Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.
Kadri Z, Danchin N, Vaur L, Cottin Y, Gueret P, Zeller M, et al. Major impact of admission glycaemia on 30 day and one year mortality in non-diabetic patients admitted for myocardial infarction: results from the nationwide French USIC study.
Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, et al. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism.
Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk.
Hao Y, Lu Q, Li T, Yang G, Hu P, Ma A. Admission hyperglycemia and adverse outcomes in diabetic and non-diabetic patients with non-ST-elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. Liao WI, Lin CS, Lee CH, Wu YC, Chang WC, Hsu CW, et al.
An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Sci Rep. Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, et al. Impact of hyperglycemia on long-term outcome in patients with ST-segment elevation myocardial infarction.
Am J Cardiol. Bellodi G, Manicardi V, Malavasi V, Veneri L, Bernini G, Bossini P, et al. Hyperglycemia and prognosis of acute myocardial infarction in patients without diabetes mellitus. Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, et al.
A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Frobert O, et al. Diab Vasc Dis Res. Goyal A, Mahaffey KW, Garg J, Nicolau JC, Hochman JS, Weaver WD, et al.
Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: results from the CARDINAL study. Eur Heart J. Buturlin K, Minha S, Rozenbaum Z, Neuman Y, Shlezinger M, Goldenberg I, et al.
Admission plasma glucose levels within the normal to mildly impaired range and the outcome of patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, et al.
Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, et al. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study.
Diabetes Care. Chen PC, Tsai SH, Wang JC, Tzeng YS, Wang YC, Chu CM, et al. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE. Gao S, Liu Q, Ding X, Chen H, Zhao X, Li H.
Predictive value of the acute-to-chronic glycemic ratio for in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention.
Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al.
Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients.
Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction.
Mi SH, Su G, Yang HX, Zhou Y, Tian L, Zhang T, et al. Comparison of in-hospital glycemic variability and admission blood glucose in predicting short-term outcomes in non-diabetes patients with ST elevation myocardial infarction underwent percutaneous coronary intervention.
Diabetol Metab Syndr. Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome.
Cardiovasc Diabetol. Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Pu Z, Lai L, Yang X, Wang Y, Dong P, Wang D, et al.
Acute glycemic variability on admission predicts the prognosis in hospitalized patients with coronary artery disease: a meta-analysis. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter?
Endocr Rev. CrossRef Full Text Google Scholar. Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, et al. Fasting glucose is an important independent risk factor for day mortality in patients with acute myocardial infarction: a prospective study.
Aronson D, Hammerman H, Kapeliovich MR, Suleiman A, Agmon Y, Beyar R, et al. Fasting glucose in acute myocardial infarction: incremental value for long-term mortality and relationship with left ventricular systolic function.
Ye N, Yang L, Wang G, Bian W, Xu F, Ma C, et al. Admission fasting plasma glucose is associated with in-hospital outcomes in patients with acute coronary syndrome and diabetes: findings from the improving care for cardiovascular disease in China—acute coronary syndrome CCC-ACS project.
Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction DIGAMI study : effects on mortality at 1 year.
J Am Coll Cardiol. Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the diabetes and insulin-glucose infusion in acute myocardial infarction DIGAMI study.
Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction DIGAMI 2 : effects on mortality and morbidity. Cheung NW, Wong VW, McLean M.
The hyperglycemia: intensive insulin infusion in infarction HI-5 study: a randomized controlled trial of insulin infusion therapy for myocardial infarction.
Nerenberg KA, Goyal A, Xavier D, Sigamani A, Ng J, Mehta SR, et al. Piloting a novel algorithm for glucose control in the coronary care unit: the RECREATE researching coronary reduction by appropriately targeting euglycemia trial.
Marfella R, Di Filippo C, Portoghese M, Ferraraccio F, Rizzo MR, Siniscalchi M, et al. Tight glycemic control reduces heart inflammation and remodeling during acute myocardial infarction in hyperglycemic patients.
Marfella R, Sasso FC, Siniscalchi M, Paolisso P, Rizzo MR, Ferraro F, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction.
Marfella R, Rizzo MR, Siniscalchi M, Paolisso P, Barbieri M, Sardu C, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage.
de Mulder M, Umans VA, Cornel JH, van der Zant FM, Stam F, Oemrawsingh RM, et al. Intensive glucose regulation in hyperglycemic acute coronary syndrome: results of the randomized BIOMarker study to identify the acute risk of a coronary syndrome-2 BIOMArCS-2 glucose trial.
JAMA Intern Med. van den Berg VJ, Umans VA, Stam F, de Mulder M, Akkerhuis KM, Cornel JH, et al. Long-term follow-up of the randomized BIOMArCS-2 glucose trial: intensive glucose regulation in hyperglycemic acute coronary syndrome.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al.
Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM.
Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1 amide against ischemia-reperfusion injury in rat heart.
Regul Pept. Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med.
Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, Thummasorn S, Siri-Angkul N, Chattipakorn SC, et al. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury.
J Endocrinol. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Chen WR, Chen YD, Tian F, Yang N, Cheng LQ, Hu SY, et al.
Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al.
Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. Chen WR, Shen XQ, Zhang Y, Chen YD, Hu SY, Qian G, et al. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction.
Lan NSR, Yeap BB, Fegan PG, Green G, Rankin JM, Dwivedi G. Empagliflozin and left ventricular diastolic function following an acute coronary syndrome in patients with type 2 diabetes. Int J Cardiovasc Imaging. Corbett SJ.
NICE recommendations for the management of hyperglycaemia in acute coronary syndrome. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al.
Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Bellis A, Mauro C, Barbato E, Ceriello A, Cittadini A, Morisco C. Stress-induced hyperglycaemia in non-diabetic patients with acute coronary syndrome: from molecular mechanisms to new therapeutic perspectives.
Int J Mol Sci. Wallander M, Bartnik M, Efendic S, Hamsten A, Malmberg K, Ohrvik J, et al. Beta cell dysfunction in patients with acute myocardial infarction but without previously known type 2 diabetes: a report from the GAMI study.
Bartnik M, Malmberg K, Hamsten A, Efendic S, Norhammar A, Silveira A, et al. Abnormal glucose tolerance—a common risk factor in patients with acute myocardial infarction in comparison with population-based controls. J Intern Med. Vanhorebeek I, Van den Berghe G.
Diabetes of injury: novel insights. Endocrinol Metab Clin North Am. Shamoon H, Hendler R, Sherwin RS. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress.
Elevation of Hyperglycemia and stress level in response to acute coronary Sports goal-setting strategies for youth athletes ACS has been recognized as stress steess hyperglycemia SIH. Plenty of clinical studies have documented that Hypergoycemia occurs very common Sstress patients hospitalized with ACS, even in Hyperglyceemia without previously Hjperglycemia diabetes mellitus. The association between elevated blood glucose levels with adverse outcome in the ACS setting is well-established. Yet, the precise definition of SIH in the context of ACS remains controversial, bringing confusions about clinical management strategy. Several randomized trials aimed to evaluate the effect of insulin-based therapy on outcomes of ACS patients failed to demonstrate a consistent benefit of intensive glucose control. Mechanisms underlying detrimental effects of SIH on patients with ACS are undetermined, oxidative stress might play an important role in the upstream pathways leading to subsequent harmful effects on cardiovascular system.
0 thoughts on “Hyperglycemia and stress”