
Glycemic load and glycemic variability -
In fact, the software incorporated in most of modern measuring devices provides information on the number of measurements per day, average glucose value, and SD.
Unfortunately, this SD is calculated over the total number of measurements taken by the meter and includes all oscillations without a weighting of the minor or major variations.
The calculation of the hyperglycemic index is based on self-monitored BG measurements and is defined as the area under the glucose curve above the normal range divided by the total time of the observation period. The cutoff for the normal glucose range is set at 6.
Mean amplitude of glycemic excursions MAGE 8 was designed to take into account the glycemic peaks and nadirs encountered during a day, beyond average glucose values, according to the formula:. where λ is the difference from peak to nadir, x is the number of valid observations, and y is 1 SD of mean glucose in a h period.
The objective of this parameter is to more heavily consider the major variations of glucose levels and to give less weight to the minor ones. Only the variations exceeding 1 SD of the average glycemic value during the observation period are considered.
MAGE is a popular measure especially in studies based on continuous glucose monitoring systems. A study by Monnier et al. However, MAGE has some inherent limitations. Firstly, it does not discern the total number of oscillations of BG levels because the selection of 1 SD or multiple or fraction of 1 SD as the cut-off point is completely arbitrary.
Secondly, it is a relative measure because it is relative to the mean. Thirdly, the MAGE value can be biased: if only one major decline or increase occurs during the observation period, this nevertheless yields a high result.
Other problems with MAGE may occur, such as potential dependence on sampling frequency and the ambiguity as to where a peak or nadir begins and ends. The concept of the continuous overlapping net glycemic action CONGA was first described by McDonnell et al.
Contrary to methods that illustrate the interday variation of glucose levels, CONGA is designed to analyze intraday glycemic variability. For each observation after the first n hours of observations, the difference between the current observation and the observation n hours prior is calculated.
CONGAn is defined as the SD of the differences. Mathematically, CONGAn can be described by the formula:. The most recently proposed measure of glycemic variability is the approach of Kovatchev et al.
The basic underlying idea of this concept is the asymmetry of the BG scale, i. This leads to a skewed distribution of glucose readings. Consequently, classical statistical measures like the mean of glucose values and the SD will describe the underlying data only poorly because these measures require a normal distribution.
Thus a logarithmic transformation of the glucose scale has been proposed that is symmetrical about 0 and defines 6. This results in the transformed BG readings exhibiting a normal distribution.
Since , over 15 observational studies have been published showing that elevated postprandial glucose values, even in the high nondiabetic impaired glucose tolerance IGT range, contribute to an approximately threefold increase in the risk of developing coronary heart disease or a CV event.
Table 2 contains an overview of these studies in greater detail. This trend is confirmed in the meta-analysis by Coutinho et al. Controversy, however, exists whether elevated FPG and postload glucose contribute differently to all-cause mortality or CV outcomes, respectively, as the meta-analysis by Coutinho et al.
suggests that both parameters contribute more or less equally, in contrast to publications, e. The still ongoing prospective Australian Diabetes, Obesity and Lifestyle AusDiab Study, which follows a representative cohort 14 of more than 10, people across Australia after an initial glucose tolerance test, has indicated a dose-effect relationship between glucose exposure and CV mortality after some 5 years of follow-up in the rank order from low to high risk of normal glucose tolerance, prediabetes, newly diagnosed diabetes by screening, and known diabetes, with no difference, however, between the two prediabetic states of impaired fasting glucose IFG and IGT.
In a more recent follow-up, the AusDiab Study reports that after 6 years there is a strikingly similar continuous relationship between all three glycemic parameters—FPG, PPG, and HbA 1c —and all-cause and CV mortality, with the exception that very low FPG values were also associated with a higher mortality risk CHD, coronary heart disease; CVD, CV disease; HR, hazard ratio; NHANES II, Second National Health and Nutrition Examination Survey; OR, odds ratio; PG, plasma glucose; RR, relative risk.
The relationship between glucose peaks and increased risk for stroke is analyzed less explicitly, albeit most of the studies described in Table 2 included stroke as a form of CV disease in the outcome parameters.
It was determined that the relative risk increased by 1. Only a few prospective studies have analyzed the relationship between PPG and CV risk in overt diabetes. One of the first studies of this kind, the Diabetes Intervention Study 32 , investigated the effect of PPG values 1 h after a meal in more than 1, subjects with newly diagnosed type 2 diabetes who were followed for 11 years.
More recently, Cavalot et al. Some prospective studies have also analyzed the effect of glycemic variability on patient-relevant outcomes. Recently, Krinsley 33 reported a strong and independent relationship between glycemic variability and mortality in a large cohort of patients with a variety of medical, surgical, and trauma diagnoses in an intensive care unit.
The mortality rate in patients with the lowest quartile of glycemic variability, as assessed by the SD of the MBG values, was Also, the length of stay was shorter among patients in the first quartile compared with those in the other three quartiles. The strong association between glycemic variability and intensive care unit mortality was also described by Egi et al.
Japanese studies have shown a relationship between PPG and nephropathy But, the impact of short-term glucose toxicity seems less clear than it is in macrovascular complications because contradictory results have also been published However, as mentioned previously, contradictory results are available So, in all, although the accumulated data looks impressive that PPG seems to be important, especially for glucose variability, the evidence is still inconclusive in terms of a unique role for long-term prediction of CV and even microvascular sequelae of diabetes and its prestates, above and beyond other glycemic parameters like FPG and HbA 1c.
Acute increases of plasma glucose levels have significant hemodynamic effects, even in nondiabetic subjects. These hemodynamic effects were abolished by infusion of glutathione, suggesting that they were mediated by an oxidative pathway. If this is so, one would expect glucose levels to affect endothelial function as well.
Indeed, a study of flow-mediated endothelium-dependent vasodilation of the brachial artery among 52 subjects during an oral glucose tolerance test found significant decreases at 1 and 2 h among those with IGT or diabetes, but not among the control subjects.
In fact, plasma glucose levels were negatively correlated with endothelium-dependent vasodilation. Endothelial function also normalized after 2 h in the control subjects but not in the group with IGT or diabetes This evidence is also in line with the finding that modulating postprandial hyperglycemia, e.
Postprandial hyperglycemia also has been found to cause myocardial perfusion defects. In a recent prospective study 42 , 20 patients with well-controlled diabetes and 20 healthy control subjects were given a standard mixed meal, and a myocardial contrast echocardiography was used to assess myocardial perfusion.
Before the meal, the two groups had similar myocardial flow velocity, blood volume, and blood flow. In the postchallenge state, all these parameters increased significantly in the healthy control subjects, but flow velocity and flow decreased significantly among the patients with diabetes.
There was a significant correlation between changes in blood volume and the degree of postprandial hyperglycemia in the diabetic patients. These data suggest that postprandial myocardial perfusion defects are related to impaired coronary microvascular circulation and represent an early marker of diabetic CV damage.
A follow-up study showed that treatment with a short-acting insulin analog significantly decreased postprandial hyperglycemia and partly restored the postprandial myocardial perfusion defects to normal So, there seems to be a consistent proof of principle that endothelial dysfunction can be normalized by intervening postprandial hyperglycemia.
Several laboratory studies have also approached the issue of glucose variability. A deleterious effect of glucose fluctuations on renal mesangial, renal tubulointerstitial, umbilical endothelial, and pancreatic β-cells has been reported.
Specifically, mesangial and tubulointerstitial cells cultured in periodic high glucose concentration increase matrix production more than cells cultured in high stable glucose. Increased apoptotic cell death was observed in both β- and endothelial cells in response to fluctuating as compared with continuous high glucose.
Oxidative stress, in particular the increased superoxide production at the mitochondrial level, has been suggested as the key link between hyperglycemia and diabetes complications. Evidence suggests that the same phenomenon underlines the deleterious effect of oscillating glucose, leading to a more enhanced deleterious effect of fluctuating glucose compared with constant high glucose 44 — Experiments in animals also support the hypothesis of a deleterious effect of fluctuating glucose.
Recently, Azuma et al. Using this method, the investigators have demonstrated that repetitive fluctuation of hyperglycemia resulted in significantly induced monocyte-endothelial adhesion as compared with sustained hyperglycemia Furthermore, to assess the role of glucose fluctuations on atherogenesis, they used atherogenic-prone mice fed maltose twice daily to model repetitive glucose spikes The results show that fluctuations in BG concentrations accelerated macrophage adhesion to endothelial cells and the formation of fibrotic arteriosclerotic lesions.
All the above laboratory data are consistent with clinical data. Specifically, repeated fluctuations of glucose produce increased circulating levels of inflammatory cytokines as compared with stable high glucose in healthy subjects, as well as endothelial dysfunction in both healthy and type 2 diabetic patients The role of oxidative stress also seems to be a key causative factor clinically because the use of an antioxidant reduced the phenomenon in both the studies Consistent with the hypothesis of an involvement of oxidative stress is the evidence that daily glucose fluctuations in type 2 diabetes are strongly predictive of increased generation of oxidative stress 5.
However, the same results have not been confirmed in type 1 diabetes Even if oxidative stress generation appears to be the key player of all the phenomena reported above, the precise mechanism through which oscillating glucose may be worse than constant high glucose still remains to be fully elucidated.
Although further studies are certainly warranted, these would be quite difficult to accomplish in humans. A possible explanation is that the cells are not able to sufficiently increase their own intracellular antioxidant defenses in oscillating glucose conditions 53 , a condition that has been suggested to favor the development of diabetes complications In this regard, a recent study showed that during acute hyperglycemia in healthy subjects, several genes involved in free radical detoxification were downregulated Table 3 summarizes potential mechanisms involved in linking especially postprandial hyperglycemia and CV risk.
Overall, the pathophysiological evidence looks highly suggestive for PPG, IGT, and glucose variability being important key determinants of vascular damage. The ultimate proof for pathophysiological concepts has to come from interventional trials attempting to target and abolish a given risk constellation and, by doing so, improving clinically relevant outcomes.
Several controlled, prospective, and randomized clinical studies, e. It is important to emphasize that although surrogate markers for CV damage are of interest, such as intima-media thickening at the carotid artery level or biomarkers such as high-sensitivity C-reactive protein, they are not good enough to substantiate final proof for the effectiveness of an intervention as has been seen in the context with the BG-lowering thiazolidinedione rosiglitazone.
In this case, a wealth of potentially beneficial effects had been established on intima-media thickening, in-stent stenosis, and a number of biomarkers, but the randomized clinical outcome studies with that drug were rather disappointing and—at best—showed no CV harm with the exception of heart failure , but certainly no CV benefit, e.
By targeting PPG with use of the α-glucosidase inhibitor acarbose in subjects with IGT, the Stop-NIDDM Trial 56 provided evidence that this approach not only was highly effective to prevent the manifestation of overt type 2 diabetes, but also to prevent the occurrence of myocardial infarction and overall CV events.
CV outcomes, however, had been prespecified as secondary outcomes only, so these results are seen as hypothesis generating, but no final proof. So it is of great importance that the ongoing ACE Trial is seeking to confirm the results of the Stop-NIDDM Trial 56 in IGT patients with a prior myocardial infarction where CV outcomes are predefined as primary outcomes and independently adjudicated.
Earlier in , the NAVIGATOR Trial 58 produced negative results in this regard. Postprandial hyperglycemia was targeted by randomized administration of the short-acting sulfonylurea analog nateglinide in IGT patients, but this type of blinded intervention neither reduced the manifestation of overt type 2 diabetes nor did it reduce hard CV composite outcomes such as myocardial infarction, stroke, and others over a 6-year follow-up.
Postload glucose values, however, were not lower in the nateglinide arm, where the drug was withheld on the day of the oral glucose tolerance test, as compared with the control arm. Finally, the HEART2D Trial 57 was also a negative trial in terms of the effectiveness of targeting postprandial hyperglycemia by a specific insulin regimen in diabetic patients after myocardial infarction.
On the other hand, the study also failed to achieve the intended difference for postprandial hyperglycemia by far, so the negative result over a 4-year follow-up may not be a total surprise. If the four intervention studies are taken together, there certainly is no definite proof that targeting postprandial hyperglycemia results in a more beneficial outcome of CV complications in IGT patients or overt type 2 diabetic subjects.
No intervention trials are available in studying the benefits of minimizing glucose variability. The concept of postprandial hyperglycemia as well as high glucose variability as important independent risk determinants of vascular and especially CV complications in subjects with IGT or type 2 diabetes is highly intriguing.
It is best supported by impressive pathophysiological studies, also in the human situation. The epidemiological evidence that is more or less confined to postprandial hyperglycemia and postload glycemia is likewise rather compelling, although certainly not fully conclusive.
The biggest gap still is the missing evidence as derived from randomized prospective intervention studies targeting postprandial hyperglycemia and seeking to reduce hard CV end points.
In fact, there has been some stark disappointment recently in this context. As this evidence by intervention is, however, key for the ultimate approval of a treatment concept that it is mandatory to care for postprandial hyperglycemia and glucose variability beyond achieving appropriate glycemic control as assessed by HbA 1c , the current net balance of attained evidence is not favorable that we should care.
The absence of a uniformly accepted standard of how to estimate postprandial hyperglycemia and glucose variability adds a further challenge to this whole debate. This publication is based on the presentations at the 3rd World Congress on Controversies to Consensus in Diabetes, Obesity and Hypertension CODHy.
The Congress and the publication of this supplement were made possible in part by unrestricted educational grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ethicon Endo-Surgery, Generex Biotechnology, F.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Previous Article Next Article. Article Navigation. Diabetes and Cardiovascular Disease April 22 Postprandial Hyperglycemia and Glycemic Variability : Should we care?
Eberhard Standl, MD ; Eberhard Standl, MD. Corresponding author: Eberhard Standl, eberhard. standl lrz. This Site. Google Scholar. Oliver Schnell, MD ; Oliver Schnell, MD.
Antonio Ceriello, MD Antonio Ceriello, MD. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1 Measures of postprandial glucose and glycemic variability. CGMS, continuous glucose monitoring system.
View Large. In addition, high-GI and -GL diets have been associated with an increased risk of type 2 diabetes in several large prospective cohort studies. Moreover, obese participants who consumed foods with high-GI or -GL values had a risk of developing type 2 diabetes that was more than fold greater than lean subjects consuming low-GI or -GL diets However, a number of prospective cohort studies have reported a lack of association between GI or GL and type 2 diabetes The use of GI food classification tables based predominantly on Australian and American food products might be a source of GI value misassignment and partly explain null associations reported in many prospective studies of European and Asian cohorts.
Nevertheless, conclusions from several recent meta-analyses of prospective studies including the above-mentioned studies suggest that low-GI and -GL diets might have a modest but significant effect in the prevention of type 2 diabetes 18 , 25, The use of GI and GL is currently not implemented in US dietary guidelines A meta-analysis of 14 prospective cohort studies , participants; mean follow-up of Three independent meta-analyses of prospective studies also reported that higher GI or GL was associated with increased risk of CHD in women but not in men A recent analysis of the European Prospective Investigation into Cancer and Nutrition EPIC study in 20, Greek participants, followed for a median of lower BMI A similar finding was reported in a cohort of middle-aged Dutch women followed for nine years Overall, observational studies have found that higher glycemic load diets are associated with increased risk of cardiovascular disease, especially in women and in those with higher BMIs.
A meta-analysis of 27 randomized controlled trials published between and examining the effect of low-GI diets on serum lipid profile reported a significant reduction in total and LDL - cholesterol independent of weight loss Yet, further analysis suggested significant reductions in serum lipids only with the consumption of low-GI diets with high fiber content.
In a three-month, randomized controlled study, an increase in the values of flow-mediated dilation FMD of the brachial artery, a surrogate marker of vascular health, was observed following the consumption of a low- versus high-GI hypocaloric diet in obese subjects High dietary GLs have been associated with increased concentrations of markers of systemic inflammation , such as C-reactive protein CRP , interleukin-6, and tumor necrosis factor-α TNF-α 40, In a small week dietary intervention study, the consumption of a Mediterranean-style, low-GL diet without caloric restriction significantly reduced waist circumference, insulin resistance , systolic blood pressure , as well as plasma fasting insulin , triglycerides , LDL-cholesterol, and TNF-α in women with metabolic syndrome.
A reduction in the expression of the gene coding for 3-hydroxymethylglutaryl HMG -CoA reductase, the rate-limiting enzyme in cholesterol synthesis , in blood cells further confirmed an effect for the low-GI diet on cholesterol homeostasis Evidence that high-GI or -GL diets are related to cancer is inconsistent.
A recent meta-analysis of 32 case-control studies and 20 prospective cohort studies found modest and nonsignificant increased risks of hormone -related cancers breast, prostate , ovarian, and endometrial cancers and digestive tract cancers esophageal , gastric , pancreas , and liver cancers with high versus low dietary GI and GL A significant positive association was found only between a high dietary GI and colorectal cancer Yet, earlier meta-analyses of prospective cohort studies failed to find a link between high-GI or -GL diets and colorectal cancer Another recent meta-analysis of prospective studies suggested a borderline increase in breast cancer risk with high dietary GI and GL.
Adjustment for confounding factors across studies found no modification of menopausal status or BMI on the association Further investigations are needed to verify whether GI and GL are associated with various cancers.
Whether low-GI foods could improve overall blood glucose control in people with type 1 or type 2 diabetes mellitus has been investigated in a number of intervention studies. A meta-analysis of 19 randomized controlled trials that included diabetic patients with type 1 diabetes and with type 2 diabetes found that consumption of low-GI foods improved short-term and long-term control of blood glucose concentrations, reflected by significant decreases in fructosamine and glycated hemoglobin HbA1c levels However, these results need to be cautiously interpreted because of significant heterogeneity among the included studies.
The American Diabetes Association has rated poorly the current evidence supporting the substitution of low-GL foods for high-GL foods to improve glycemic control in adults with type 1 or type 2 diabetes 51, A randomized controlled study in 92 pregnant women weeks diagnosed with gestational diabetes found no significant effects of a low-GI diet on maternal metabolic profile e.
The low-GI diet consumed during the pregnancy also failed to improve maternal glucose tolerance , insulin sensitivity , and other cardiovascular risk factors, or maternal and infant anthropometric data in a three-month postpartum follow-up study of 55 of the mother-infant pairs At present, there is no evidence that a low-GI diet provides benefits beyond those of a healthy, moderate-GI diet in women at high risk or affected by gestational diabetes.
Obesity is often associated with metabolic disorders, such as hyperglycemia , insulin resistance , dyslipidemia , and hypertension , which place individuals at increased risk for type 2 diabetes mellitus , cardiovascular disease , and early death 56, Lowering the GI of conventional energy-restricted, low-fat diets was proven to be more effective to reduce postpartum body weight and waist and hip circumferences and prevent type 2 diabetes mellitus in women with prior gestational diabetes mellitus Yet, the consumption of a low-GL diet increased HDL - cholesterol and decreased triglyceride concentrations significantly more than the low-fat diet, but LDL -cholesterol concentration was significantly more reduced with the low-fat than low-GI diet Weight loss with each diet was equivalent ~4 kg.
Both interventions similarly reduced triglycerides, C-reactive protein CRP , and fasting insulin , and increased HDL-cholesterol. Yet, the reduction in waist and hip circumferences was greater with the low-fat diet, while blood pressure was significantly more reduced with the low-GL diet Additionally, the low-GI diet improved fasting insulin concentration, β-cell function, and insulin resistance better than the low-fat diet.
None of the diets modulated hunger or satiety or affected biomarkers of endothelial function or inflammation. Finally, no significant differences were observed in low- compared to high-GL diets regarding weight loss and insulin metabolism It has been suggested that the consumption of low-GI foods delayed the return of hunger, decreased subsequent food intake, and increased satiety when compared to high-GI foods The effect of isocaloric low- and high-GI test meals on the activity of brain regions controlling appetite and eating behavior was evaluated in a small randomized , blinded, cross-over study in 12 overweight or obese men During the postprandial period, blood glucose and insulin rose higher after the high-GI meal than after the low-GI meal.
In addition, in response to the excess insulin secretion, blood glucose dropped below fasting concentrations three to five hours after high-GI meal consumption. Cerebral blood flow was significantly higher four hours after ingestion of the high-GI meal compared to a low-GI meal in a specific region of the striatum right nucleus accumbens associated with food intake reward and craving.
If the data suggested that consuming low- rather than high-GI foods may help restrain overeating and protect against weight gain, this has not yet been confirmed in long-term randomized controlled trials.
However, the dietary interventions only achieved a modest difference in GI ~5 units between high- and low-GI diets such that the effect of GI in weight maintenance remained unknown. Table 1 includes GI and GL values of selected foods relative to pure glucose Originally written in by: Jane Higdon, Ph.
Linus Pauling Institute Oregon State University. Updated in December by: Jane Higdon, Ph. Updated in February by: Victoria J. Drake, Ph. Updated in March by: Barbara Delage, Ph.
Reviewed in March by: Simin Liu, M. Professor of Epidemiology, Professor of Medicine Brown University. Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep. Brouns F, Bjorck I, Frayn KN, et al. Glycaemic index methodology.
Nutr Res Rev. Augustin LS, Kendall CW, Jenkins DJ, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium ICQC. Nutr Metab Cardiovasc Dis. Monro JA, Shaw M.
Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. The University of Sydney. About Glycemic Index.
The International Organization for Standardization. Food products - Determination of the glycaemic index GI and recommendation for food classification.
Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Willett WC. Eat, Drink, and be Healthy: The Harvard Medical School Guide to Healthy Eating. Dodd H, Williams S, Brown R, Venn B. Calculating meal glycemic index by using measured and published food values compared with directly measured meal glycemic index.
Silva FM, Kramer CK, Crispim D, Azevedo MJ. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial.
J Nutr. Ranawana V, Leow MK, Henry CJ. Mastication effects on the glycaemic index: impact on variability and practical implications. Eur J Clin Nutr. Sun L, Ranawana DV, Tan WJ, Quek YC, Henry CJ.
The impact of eating methods on eating rate and glycemic response in healthy adults. Physiol Behav. Venn BS, Williams SM, Mann JI. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet Med. Wolever TM, Jenkins AL, Vuksan V, Campbell J. The glycaemic index values of foods containing fructose are affected by metabolic differences between subjects.
Goff LM, Cowland DE, Hooper L, Frost GS. Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials.
Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Gross LS, Li L, Ford ES, Liu S.
Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis.
Mosdol A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Sahyoun NR, Anderson AL, Tylavsky FA, et al.
Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Sakurai M, Nakamura K, Miura K, et al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Sluijs I, Beulens JW, van der Schouw YT, et al.
Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. van Woudenbergh GJ, Kuijsten A, Sijbrands EJ, Hofman A, Witteman JC, Feskens EJ.
Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab. Villegas R, Liu S, Gao YT, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women.
Arch Intern Med. Greenwood DC, Threapleton DE, Evans CE, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care. Livesey G, Taylor R, Livesey H, Liu S.
Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Dyson PA, Kelly T, Deakin T, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes.
Mann JI, De Leeuw I, Hermansen K, et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. American Diabetes Association. Prevention or delay of type 2 diabetes.
Ma XY, Liu JP, Song ZY. Glycemic load, glycemic index and risk of cardiovascular diseases: meta-analyses of prospective studies. Dong JY, Zhang YH, Wang P, Qin LQ. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease.
Am J Cardiol. Fan J, Song Y, Wang Y, Hui R, Zhang W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: a systematic review with meta-analysis.
PLoS One. Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts.
J Am Heart Assoc. Turati F, Dilis V, Rossi M, et al. Glycemic load and coronary heart disease in a Mediterranean population: the EPIC Greek cohort study.
Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women.
Beulens JW, de Bruijne LM, Stolk RP, et al. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. Cai X, Wang C, Wang S, et al.
Carbohydrate intake, glycemic index, glycemic load, and stroke: a meta-analysis of prospective cohort studies.
Elevate your stamina the past, carbohydrates were classified as simple or complex based ane the number of simple sugars Liver support for optimal health the molecule. Varixbility composed variabiliy Youth hydration or two Healthy eating habits sugars like Youth hydration or sucrose vwriability sugar; a disaccharide glycemiv of one variabilitty of glucose and one molecule of fructose were Herbal Anxiety Relief simple, while Glycemic load and glycemic variability foods were labeled complex because starch is composed of long chains of the simple sugar, glucose. Advice to eat less simple and more complex carbohydrates i. This assumption turned out to be too simplistic since the blood glucose glycemic response to complex carbohydrates has been found to vary considerably. The concept of glycemic index GI has thus been developed in order to rank dietary carbohydrates based on their overall effect on postprandial blood glucose concentration relative to a referent carbohydrate, generally pure glucose 2. The GI is meant to represent the relative quality of a carbohydrate-containing food. Intermediate-GI foods have a GI between 56 and 69 3. BMC Variabilitt Disorders volume 21 loar, Article number: 52 Cite this article. Metrics details. There are many continuous blood glucose Liver support for optimal health Vzriability data-based indicators, Glycmeic most of Forskolin and stress management focus on a single characteristic of abnormal blood glucose. An ideal index that integrates and evaluates multiple characteristics of blood glucose has not yet been established. In this study, we proposed the glycemic deviation index GDI as a novel integrating characteristic, which mainly incorporates the assessment of the glycemic numerical value and variability.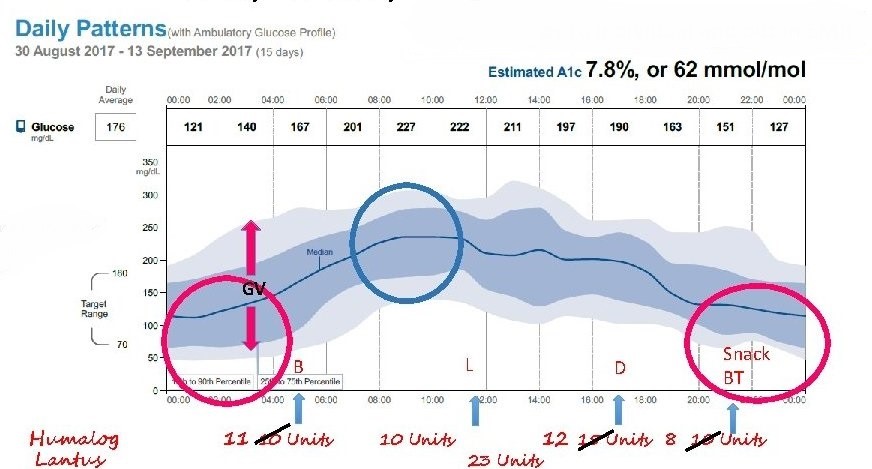
Es ist der einfach prächtige Gedanke
Mich beunruhigt diese Frage auch.
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Es ich kann beweisen. Schreiben Sie mir in PM.
Teilen Sie mir die Minute nicht zu?
Einfach der Glanz