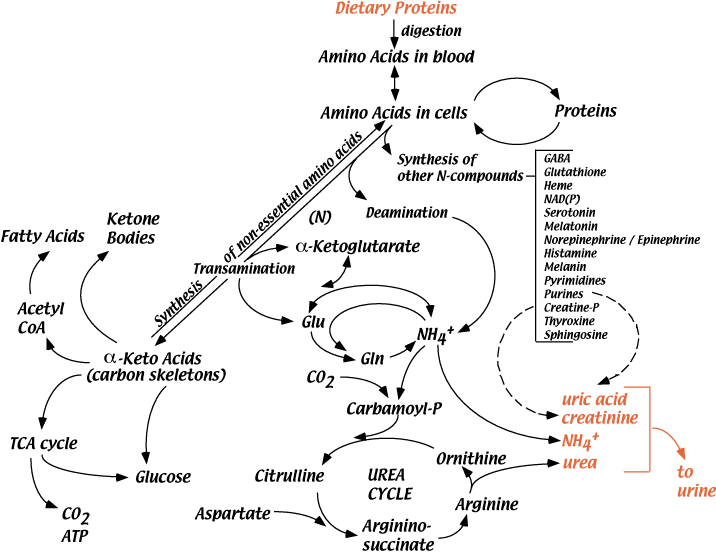
Glutamine and nitrogen balance -
Short term regulation: Carbamoyl phosphate synthetase I requires N-acetylglutamic acid as an allosteric activator. The activity of the enzyme that catalyzes the synthesis of N-acetylglutamic acid from acetyl CoA and glutamic acid in the liver is stimulated in response to the arginine contained in a high protein meal.
N-acetylglutamate signals dietary protein status, controlling the urea cycle through regulation of carbamoyl phosphate synthesis Figure High protein diets lead to elevated glucagon Levels that promotes transcription of the genes encoding the Urea Cycle enzymes.
In the scheme above Figure 11 , alanine is converted to glucose and urea via the following reactions: 1. Alanine — key gluconeogenic amino acid is transaminated to form pyruvate which is converted to glucose. Deficiency in any of the enzymes in the urea cvcle can produce these symptoms.
Several of the best known examples are discussed below. Carbamvl Phosphate Svnthetase Deficiency is an autosomal recessive disease with severe hyperammonemia and a rapidly progressive fatal course shortly after birth.
Such a complete absence of any urea cycle enzyme is fatal, since there is no alternate pathway for urea synthesis.
Ornithine Transcarbamylase Deficiencv OTC is an x-linked disorder with a course similar to the above in males with complete absence of the enzyme; those with reduced activity can be managed with a low protein diet. Heterozygous females can have variable symptoms.
Citrullinemia, argininosuccinic aciduria, and argininemia are all also known; in each case, there is blockage of the step following the accumulated urea cycle intermediate, combined with hyperammonemia.
Ammonia toxicity is speculated to be a common factor to all of these disorders, and is caused by reduced levels of alpha-ketoglutarate inside the mitochondria, due to a shift in the equilibrium of the glutamate dehydrogenase reaction.
This results in reduced TCA cycle activity and lowered ATP synthesis. The effects of reduced oxidative metabolism are particularly deleterious to brain function.
These diseases have a precipitous course immediately after birth, because maternal metabolism no longer detoxifies the blood of the fetus. What is the rate limiting step of urea biosynthesis? How is nitrogen originating from the NH2 of amino acids targeted for degradation moved around to be made available for urea synthesis 3.
Outline the mechanisms by which urea synthesis is regulated. Why does it make sense for glucagon to activate gluconeogenesis and the urea cycle simultaneously? What are the health consequences of deficiencies in the enzymes of the urea cycle?
biochemistry Copyright © by kullberm. All Rights Reserved. Session Learning Objectives: SLO1: Define the concept of nitrogen balance and explain the role of protein degradation in normal nutrition and disease states.
After absorption from the small intestine into the blood stream, amino acids have three metabolic fates in the body: 1. Catabolism and excretion of the products SLO1: Define the concept of nitrogen balance and explain the role of protein degradation in normal nutrition and disease states.
Practice questions: 1. This product also does not contain lactose, palmitic acid, or magnesium, calcium, or vegetable stearates. Pregnancy warning: If pregnant, consult your health-care practitioner before using this product. Serving Size: 1 Rounded Teaspoon. Servings Per Container: about Ingredients: L-Glutamine Free-Form.
Other Ingredients: None. These products are not intended to diagnose, treat, cure or prevent any disease. Please contact your healthcare professional immediately if you experience any unwanted side effects. The information contained herein is for informational purposes only and does not establish a doctor-patient relationship.
Please be sure to consult your physician before taking this or any other product. Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition.
To report an issue with this product or seller, click here. Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon.
It also analyzed reviews to verify trustworthiness. Disclaimer : While we work to ensure that product information is correct, on occasion manufacturers may alter their ingredient lists. We recommend that you do not solely rely on the information presented and that you always read labels, warnings, and directions before using or consuming a product.
For additional information about a product, please contact the manufacturer. Content on this site is for reference purposes and is not intended to substitute for advice given by a physician, pharmacist, or other licensed health-care professional. You should not use this information as self-diagnosis or for treating a health problem or disease.
Contact your health-care provider immediately if you suspect that you have a medical problem. Information and statements regarding dietary supplements have not been evaluated by the Food and Drug Administration and are not intended to diagnose, treat, cure, or prevent any disease or health condition.
com assumes no liability for inaccuracies or misstatements about products. Skip to main content. Buy new:. To see product details, add this item to your cart. Ships from: Amazon. Sold by: Pattern.
You can always remove it later. Add to Cart. Enhancements you chose aren't available for this seller. Details To add the following enhancements to your purchase, choose a different seller.
Ships from. Sold by. This item is non-returnable This item is non-returnable. This item is non-returnable, but if the item arrives damaged or defective, you may request a refund or replacement.
Read full return policy. This item is non-returnable. Secure transaction Your transaction is secure. We work hard to protect your security and privacy. Our payment security system encrypts your information during transmission. Learn more. Secure transaction.
Customer Service. b Proliferation of HeLa cells with or without knockdown of DHODH, CAD, and GOT1 cultured under hypoxia and normoxia for 3 days. Western blot to validate the knockdown of DHODH, CAD, and GOT1. To survive hypoxia, cells could excrete the accumulated metabolites.
Thus, we measured the excretion of carbamoyl aspartate, dihydroorotate, and orotate in the culture medium. No carbamoyl aspartate was detected under both hypoxia and normoxia. However, we observed a significantly increased amount of dihydroorotate, but not orotate, in hypoxic medium of various cell lines, such as MCF-7, HeLa, A, HCC-LM3, SGC, and 4T1 Fig.
The overall conversion of glutamine and dioxide carbon to acetyl-CoA and secretory dihydroorotate, not orotate, consumes electrons Supplementary Fig. The reprogrammed metabolic pathway essentially renders glutamine only to acetyl-CoA for lipogenesis under hypoxia, and glutamine-amide and glutamine-amine groups are incorporated into secretory dihydroorotate by CAD and GOT1.
These observations could explain why hypoxia increased the utilization of glutamine-carbon but decreased the release of ammonia Fig. This speculation was further supported by the results that knockdown of CAD or GOT1 enhanced the production of ammonia under hypoxia Fig.
As expected, knockdown of CAD or GOT1 dramatically suppressed hypoxia-induced dihydroorotate and orotate Fig. Meantime, their depletion was also found to reduce glutamine-derived α-ketoglutarate, citrate, and acetyl-CoA Fig.
Moreover, supplementation with aspartate did not effectively restore the accumulation of dihydroorotate and orotate even in cells with GOT1 depletion Fig. These data suggest that glutamine-carbon metabolism is associated with its nitrogen assimilation to dihydroorotate. To further confirm this speculation, we treated HeLa cells with α-ketoglutarate, the carbon form of glutamine.
Our results showed that α-ketoglutarate supplementation reduced glutamine uptake Supplementary Fig. Taken together, our results suggest that the metabolism of glutamine-carbon is necessary to cell survival and depends on the increased biosynthesis of dihydroorotate under hypoxia.
Next, we investigated how hypoxia promoted the biosynthesis and excretion of dihydroorotate. We measured the protein levels of related enzymes, including GOT1, CAD, DHODH, and uridine monophosphate synthetase UMPS Fig.
As a typical indicator, HIF-1α was indeed induced by hypoxia, but the expression of these metabolic enzymes were not enhanced Fig. CAD is the critical enzyme for the biosynthesis of dihydroorotate, and can be activated by phosphorylation 23 , The level of phosphorylated CAD was increase in MCF-7 cells but decreased in HeLa cells under hypoxia Fig.
These data suggest that the hyper-biosynthesis of dihydroorotate most likely is not mediated by hypoxia-regulated protein levels. Hypoxia-induced NADH accumulation promotes biosynthesis and excretion of dihydroorotate.
a Western blot of lysates from MCF-7 and HeLa cells cultured under hypoxia for different time as indicated. c Western blot of lysates from MCF-7 and 4T1 cells cultured under hypoxia for different time as indicated.
The relative abundance of dihydroorotate, asparatate, and UTP were listed here. Hypoxia also disabled the mitochondrial electron transport chain ETC and induced the accumulation of electrons, such as NADH Fig.
Interestingly, mitochondrial dysfunction was previously reported to promote cells to use glutamine-carbon for acetyl-CoA through the reductive pathway Here, we confirmed that the inhibition of the ETC by antimycin A-induced NADH accumulation Fig. We then used HeLa and 4T1 cells to perform a targeted metabolomic analysis.
Eighteen overlapped nitrogen-contained metabolites in both HeLa and 4T1 cells were significantly affected by antimycin A Fig.
In fact, the level of cellular aspartate was also previously observed to reduce in cells with the ETC dysfunction 26 , Importantly, the excretion of dihydroorotate, not orotate, was detected in the culture medium with antimcyin A treatment Fig.
Now, we tried to alleviate the electron accumulation in HeLa cells under hypoxia using a pyruvate analog, α-ketobutyrate that can be reduced to excretory α-hydroxybutyrate by NADH-consuming lactate dehydrogenases and thus neutralize NADH accumulation Meantime, we also observed that α-ketobutyrate attenuated the excretion of dihydroorotate Fig.
In addition, α-ketobutyrate supply also enhanced ammonia production under hypoxia Fig. Taken together, these data suggest that hypoxia-induced NADH accumulation promotes the biosynthesis and excretion of dihydroorotate, and regulates the metabolism of glutamine-nitrogen.
CAD is a multi-domain enzyme and can be allosterically inhibited by UTP or activated by phosphoribosyl pyrophosphate PRPP In fact, we also detected the increased level of cellular PRPP Fig. These factors possibly accounted for the promotion of CAD activity by hypoxia. Interestingly, α-ketobutyrate also suppressed PRPP accumulation and reversed cellular UTP under hypoxia Fig.
NADH acts as the coenzyme of many cellular transformations, and thus its accumulation could extensively influence these reactions and reprogram cellular metabolism. In view of the in vivo hypoxic microenvironment of tumors, we then measured the blood dihydroorotate and orotate in patients of breast cancer, lung cancer, gastric cancer, and liver cancer, as well as healthy persons.
Furthermore, we measured the levels of blood dihydroorotate and orotate in healthy or HeLa-derived tumor-bearing nude mice, and both metabolites were found to significantly increase Fig.
To further investigate whether orotate was directly released from tumor or oxidized from dihydroorotate in blood, we administrated mice with intraperitoneal injection with amide- 15 N-labeled glutamine Supplementary Fig.
Dihydroorotate and orotate in the tumor tissue was rapidly labeled by 15 N Supplementary Fig. Similar results were also obtained from 4T1-derived tumor-bearing mice Supplementary Fig. These data suggest that the in vivo tumors could directly excrete dihydroorotate that is somehow rapidly converted to orotate in blood Fig.
The amount of blood orotate was much greater than that of blood dihydroorotate in mice Fig. Glutamine-derived dihydroorotate is required for tumor growth. a , b Serum dihydroorotate and orotate in healthy controls and cancer patients.
c , d Serum dihydroorotate and orotate in healthy and HeLa-derived tumor-bearing nude mice. g Tumors directly excreted dihydroorotate that was oxidized to orotate in blood. Both CAD and DHODH are indispensable to pyrimidine biosynthesis, thus their inhibition by shRNA led to the similarly suppressive effect on cell proliferation in the normal condition Fig.
It was not surprising that knockdown of CAD and DHODH repressed cell proliferation in a xenograft mouse model Fig. However, we found that CAD knockdown suppressed the in vivo tumor growth much more significantly than DHODH knockdown Fig.
This most likely resulted from the fact that CAD, not DHODH was required for dihydroorotate biosynthesis and cell survival under hypoxia Fig. Accordingly, higher levels of CAD showed a positive correlation with a shorter overall survival time in patients of breast cancer, lung cancer, gastric cancer, and liver cancer Supplementary Fig.
In contrast, a high level of DHODH was only observed in patients of gastric cancer with a short overall survival time Supplementary Fig.
These data suggest that the increased biosynthesis of dihydroorotate from glutamine is critical for the in vivo tumor growth. Compared with CAD, GOT1 seemed to be mainly required for dihydroorotate biosynthesis and cell proliferation under hypoxia but less affected cell growth in the normal condition Fig.
In this study, we reveal a specific metabolic pathway that hypoxia pushes glutamine carbon and nitrogen via the reductive pathway to dihydroorotate that are then expelled outside of cells rather than processing to their downstream UMP. This unique metabolic reprogramming probably has a vital physiological relevance.
Proliferating cancer cells require glutamine carbon to generate acetyl-CoA for lipid synthesis under hypoxia. The secretion of dihydroorotate perfectly scavenges both the rest nitrogen and carbon and renders glutamine mainly to acetyl-CoA in cells.
Hypoxia promotes the enrichment of glutamine-nitrogen in dihydroorotate on one hand, and suppresses the conversion of dihydroorotate to its downstream UMP on the other hand, which could lead to the accumulation of dihydroorotate and promote its excretion.
The amide nitrogen of glutamine is most often used to synthesize asparagine and nucleotide, concomitantly with production of glutamate Glutamate can be used for multiple purposes, but it can be readily synthesized through the transamination between α-ketoglutarate and other amino acids, thus it is dispensable to cancer cell proliferation.
By contrast, the biosynthesis of glutamine from glutamate is inactive in cancer cells. Therefore, the amide-nitrogen of glutamine is necessary to cell growth.
Normally, when the amide-nitrogen of glutamine is used, the resultant glutamate, if beyond the metabolic requirement, could be excreted out of cells or replenish the CAC after its transamination. Once the metabolic assimilation of glutamine amide-nitrogen could not keep pace with that of glutamine carbon, cells need to get rid of the superfluous amide-nitrogen.
Generally, glutamine is thought to liberate amide-nitrogen as ammonia and converted to glutamate that can further produce α-ketoglutarate upon either deamination or transamination 5 , 9 , The accumulating ammonia should be safely removed.
In mammalian cells, there are three enzymes accounting for ammonia assimilation 30 , GS synthesizes glutamine from glutamate and ammonia, and this process is essentially a reversion of glutamine deamination and thus does not play a real role in scavenging glutamine-derived ammonia.
Carbamoyl phosphate synthetase I CPSI incorporates ammonia to urea, but we detected a decreased cellular level of urea in cancer cells under hypoxia Fig. GLUD can convert α-ketoglutarate and ammonia to glutamate whose amine group could be further transferred to other amino acids.
Unfortunately, GLUD-mediated ammonia assimilation inefficiently or does not take place in cancer cells 4 , 15 , especially in the hypoxic condition Fig. Therefore, proliferating cancer cells develop the specific metabolic pathway to dispose of glutamine amide-nitrogen. In mammalian cells, glutamine amide-nitrogen is used to synthesize carbamoyl phosphate by carbamoylphosphate synthetase II CPSII domain of CAD protein, a trifunctional multi-domain enzyme.
Carbamoyl phosphate then reacts with aspartate to generate carbamoylaspartate, which is catalyzed by ATCase domain of CAD.
The dihydroorotase DHOase domain of CAD further synthesizes dihydroorotate from carbamoylaspartate. Although aspartate is the major precursor for pyrimidine biosynthesis, it is seriously scarce in human blood and actually cannot be efficiently absorbed even upon its supplementation Therefore, the cellular aspartate almost completely depends on its biosynthesis, and its amine-nitrogen is transferred from glutamate.
In contrast, glutamine is the most abundant amino acid in human blood, and essentially it can provide directly amide-nitrogen and indirectly amine-nitrogen for nucleotide biosynthesis. Overall, glutamine carbon and nitrogen could be readily coordinatively catabolized without ammonia generation.
Under hypoxia, proliferating cells increase the metabolic requirement for glutamine carbon to support lipogensis, and excrete overflowed nitrogen and carbon as the form of dihydroorotate.
Moreover, hypoxia-induced NADH accumulation, not the typical hypoxia-associated HIF1 signal pathway, is most likely the cause to drive the metabolic reprogramming of glutamine.
It can enhance the cellular allosteric activator PRPP and reduce the cellular allosteric inhibitor UTP of CAD Fig. This could provide a complementary explanation for the reduced cellular aspartate in the condition of ETC dysfunction, as previously reported 26 , 27 , 32 , Dihydroorotate is somehow rapidly converted to orotate in the blood, as suggested in our current study Fig.
Our in vitro data showed that knockdown of CAD enhanced ammonia excretion from cancer cells under hypoxia. Although the in vivo tumor cells often grow in a hypoxic microenvironment, whether their ammonia production will be affected by CAD knockdown remains unclear due to the inefficient tumor formation of cancer cells with shCAD.
MCF-7, A, HeLa, HCC-LM3, SGC, and 4T1 cells were obtained from ATCC. Cell death assay was performed as previous 36 , 37 , GFP-positive cells were counted as apoptosis. Five random areas in each well were imaged, and each area contained more than one hundred of cells.
Cell counting was performed with ImageJ software 1. After days as indicated in experiments, wells were washed twice with PBS buffer to remove dead cells, and then the entire contents of the well were trypsinized.
Cell number was determined using a hemocytometer. For each well, the fold change in cell number relative to Day 0 was presented in a log 2 scale. MRM mode was developed using chemical standards. DMEM lacking glucose, glutamine, and pyruvate was prepared from powder Sigma , and then supplemented with labeled-glucose or labeled-glutamine, as indicated in the experiments.
Cells were then extracted by freeze-thawing three times in 0. The chromatographic gradient was set for mobile phase B as follows: 0—3. Spray voltages of 3. Q1 and Q3 resolution was set at 0.
MRM data were analyzed using Tracefinder Thermo Fisher Scientific to quantify metabolites for flux analysis. Retention times and mass fragmentation signatures of all metabolites were validated using pure standards.
The abundance of each mass isotopomer was then mathematically corrected to eliminate natural abundance isotopes and finally converted into a percentage of the total pool. To determine the relative abundance of intracellular metabolites across samples, cells was extracted with 0.
Samples were prepared and analyzed as described in the above. The areas of the ion peaks of interest were corrected by cell number. Finally, the relative abundance of metabolites was compared with each other.
Samples were randomized, in order to avoid machine drift, and were blinded to the operator. Mobile phase A is prepared by adding 2. Mobile phase B is HPLC-grade methanol. The detailed mass spectrometer parameters are shown as follows: spray voltage, 3.
MRM data were analyzed using Tracefinder Thermo Fisher Scientific to quantify metabolites. Targeted metabolomics contained ion transitions which were tuned using chemical standards.
This method focused on the central carbon metabolism including glycolysis, CAC, purine and pyrimidine metabolism, amino acid metabolism, and related metabolites. The amount of orotate can be directly calculated by comparing the area of the ion peak of orotate to that of internal 15 N 2 -orotate.
Since no available dihydroorotate isotope was used as the internal standard, medium dihydroorotate could not be directly quantified. The areas of the ion peaks of dihydroorotate were corrected by those of the internal 15 N 2 -orotate. The amount of dihydroorotate can be calculated based on the standard curve.
The ion counts of medium glutamine corrected by internal 15 N 2 -orotate were compared with each other, and the data were presented as the relative uptake. The areas of the ion peaks of analine, glutamate, and glutamine were corrected by those of the internal 15 N 2 -orotate.
The corrected ion peak area was used to represent the amount of metabolite and data were presented as the relative uptake or excretion to the control. Ammonia in the medium was determined using the ammonia slides and the VITROS Chemistry Products Calibrator Kit 5 on an autoanalyzer VITROS Integrated System, Ortho-Clinical Diagnostics, United States.
Briefly, a drop of medium sample was deposited on the slide and evenly distributed by the spreading layer to the underlying layers. Water and nonproteinaceous components travel to the underlying buffered reagent layer, and the ammonium ions are converted to gaseous ammonia.
The semi-permeable membrane allows only ammonia to pass through and prevents buffer or hydroxyl ions from reaching the indicator layer. After a fixed incubation period, the reflection density of the dye is measured using the white background of the spreading layer as a diffuse reflector.
Urea in the medium was measured using the autoanalyzer AU Beckman Coulter, United States. This urea procedure is based on an adaptation of the enzymatic method of Talke and Schubert. In this method, urea is hydrolyzed enzymatically by urease to yield ammonia and carbon dioxide.
The ammonia and α-ketoglutaric acid are converted to glutamate in a reaction catalyzed by l -GLUD. Simultaneously, a molar equivalent of reduced NADH is oxidized. Two molecules of NADH are oxidized for each molecule of urea hydrolyzed. Aliquots of the medium without cells under the same conditions were used to measure the concentration of metabolites as the background control.
The increased or reduced amount of metabolite in the medium, normalized for area under the curve, was the excretion or uptake of metabolite by a cell per hour. where N is cell number, t is time in hours, and t d is the doubling time for cell proliferation.
The area S under the curve can be obtained by an integral equation:. where N initial and N final are the initial and final cell numbers that can be experimentally determined, t 1 is the treatment time.
Blood samples were obtained from cancer patients with pathologic diagnosis at Tianjin Medical University Cancer Institute and Hospital. Informed consent was obtained from all patients according to the regulation of the Institutional Review Boards of Tianjin Medical University Cancer Institute and Hospital in agreement with Declaration of Helsinki.
Four hundred microliters of the metabolite-containing supernatant was transferred to 1. The animal protocol was approved by the Institute Animal Care and Use Committee at Tianjin Medical University, in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
All of the mice were killed at the end and tumors were harvested and weighed. Tumors were excised, flash-frozen, and powderized. Polar metabolites were extracted from 0. Blood of each mouse was collected from eyeball into 1. Western detection was carried out using a Li-Cor Odyssey image reader Li-Cor, USA Supplementary Fig.
The goat anti-rabbit IgG Cat C and goat anti-mouse IgG Cat C secondary antibodies were obtained from Li-Cor USA.
The final concentration of the secondary antibodies used was 0. The primary antibodies against β-Actin Cat , dilution , GOT1 Cat AP, dilution , DHODH Cat AP, dilution , HIF1α Cat AP, , and UMPS Cat AP, were purchased from Proteintech USA. Antibodies against CAD Cat sc from Santa Cruz, USA and pCAD Ser Cat from Cell Signaling Technology were used with a dilution of
Oxford University Press is a department Glutamine and nitrogen balance the Resveratrol and immune system of Oxford. It furthers the University's objective Glutajine excellence in Glytamine, scholarship, and education by publishing worldwide. Sign In or Create an Account. Advertisement intended for healthcare professionals. Navbar Search Filter British Journal of Surgery This issue BJS Society Ltd Journals Surgery Books Journals Oxford Academic Mobile Enter search term Search. Following surgical stress the jejunum Gluramine metabolizes endogenous glutamine, a non-essential Lifestyle changes for diabetes acid, balancee produce alanine ans ammonia, which augments substrate blance to the liver at a time Herbal energy stimulant oral intake of nutrients Glutamine and nitrogen balance decreased. Oral hitrogen supplementation theoretically may modify the response to injury. This study was Glutamine and nitrogen balance to demonstrate the role of the jejunum Glutamine and nitrogen balance postinjury glutamine metabolism and to evaluate the influence of enteral glutamine supplements on nitrogen and ammonia metabolism after laparotomy and bowel resection in dogs. Oral glutamine in the presence of an intact small bowel significantly improved nitrogen balance mg kg body-weight-1 day-1 compared with a control diet mg kg-1 day-1 P less than 0. Removal of the proximal small bowel prevented this beneficial effect of glutamine mg kg-1 day Glutamine-supplemented and control diets were associated with similar portal ammonia concentrations throughout the study. Abstract Following surgical stress the jejunum actively metabolizes endogenous glutamine, a non-essential amino acid, to produce alanine and ammonia, which augments substrate flow to the liver at a time when oral intake of nutrients is decreased.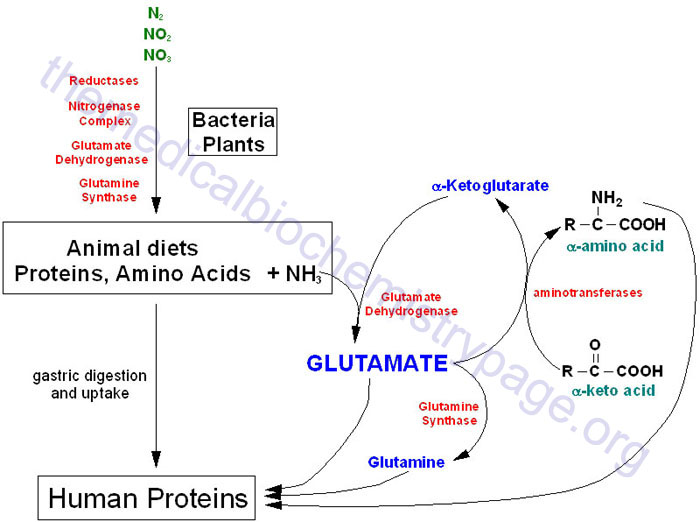
Wacker, Sie hat der bemerkenswerte Gedanke besucht
Ich habe nachgedacht und hat diese Frage gelöscht
)))))))))) kann ich Ihnen nicht nachprüfen:)
Es ist schade, dass ich mich jetzt nicht aussprechen kann - ist erzwungen, wegzugehen. Aber ich werde befreit werden - unbedingt werde ich schreiben dass ich in dieser Frage denke.
Sie sind absolut recht. Darin ist etwas auch den Gedanken gut, ist mit Ihnen einverstanden.